| Name | Value |
|---|---|
| All returns accepted | ReturnsNotAccepted |
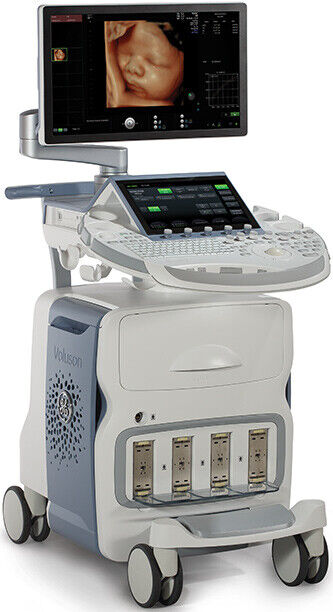
| Model | Voluson E8 BT19 |
| Country/Region of Manufacture | Austria |
| MPN | Does Not Apply |
| Brand | GE |
| Intended Use/Discipline | Obstetrics & Gynecology Radiology |
GE Voluson E8 Expert HD Live B T19 23’’ High resolution Display FullHD 15’’ High Resolution Touch Screen Color Doppler, PW Doppler, M-Mode, Angio, B-Flow, Coded Excitation (CE), Coded Excitation (CE), OPTIONS: 4D AdvanceVocal IIHd Live StudioElastographyV-SriAdvanced STICAdvanced VCRDvd & USB recordrrSono VCAD LaborCode Contrast ImagingSono AVCSono CNSAnatomical M-modeRadiant FlowTricefyFetal HQSonometrium Premiu Security FeaturesShear ElastoIETABT activation BT19 New features • SonoCNS – Automation based on AI development to help analyze the fetal brain • Slow Flow HF- Enhanced percusion of the small vessels • Radiantflow – Enhane sensitivity and improved artifact suppression to show better vessel separation & 3D • fetalHQ – intuitive tool to comprehensive evaluate size, shape and contractility of the fetal heart • Gynecology protocols with IOTA adnex assement, IETA (internal Endometrial Tumor Analysis), Scan assistant • New GS Age table Nyber • Advanced 3d printing • xTouch – intuitive user interface (finger touch gestures similar to smart devices) • PW Tissue Doppler (Tissue Velocity Imaging) for Wall Motion Analysis => Modified PW algorithm suitable for tracking tissue motion instead of blood flow. • Antegrade VTI- measurements for Pulmonary Veins • Pelvic Floor Measurements • Uterus Length Measurements • C1-6 D XD clear single crystal probe for solid penetration and very good resolution Normal 0 false false false EN-US X-NONE X-NONE Transducers RAB6-D 4D convex probeC1-6 D 2D XD Clear convex probeRIC5-9D 3D endovaginal probe. Accesories Sony Printer UP-D898MD Unit comes with 60 days warranty in parts 1 year warranty available for $6500 additional CONTACT INFORMATIONJaime Munoz JACO MEDICAL EQUIPMENT, INC 13100 Kirkham Way, Suite 204 Poway, CA 92064 FDA Regulation Notice:“The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.”




















There are no reviews yet.